
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Lecture № 30 ФХС
Theme:Application of the rule of the lever in threecomponent system. Systems MgO-Al2O3- SiO2, K2O - Al2O3- SiO2, Na2O - CaO- SiO2, Li2O - Al2O3- SiO2
In трехкомпонентной to the diagram of a condition three types of tasks with use of a rule of the lever - definition of the quantitative contents of phases formed for all way кристаллизации are possible(probable) at presence in system:
1. Liquid and one firm phase;
2. Liquid and two firm phases;
3. Definition of a quantitative parity(ratio) of firm phases allocated from расплава at the given moment кристаллизации.
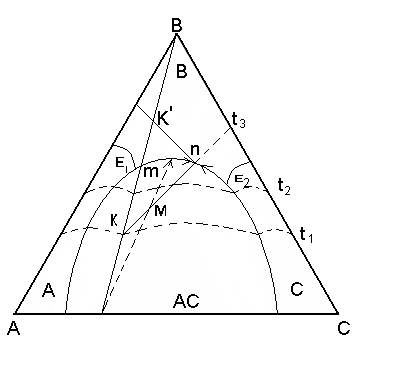
1. The point of structure of a liquid phase is in a field primary кристаллизации what - or connection. Let structure расплава М will be cooled up to temperature t 2 thus temperature in balance there is a liquid phase of structure m and crystals АС allocated during cooling initial расплава. According to a rule of the lever the contents of phases for all way кристаллизации from t1 - t2 is defined(determined) from expression:
Сод ж.ф. =
The contents крист АС =
2. The point of structure of a liquid phase is on a boundary curve. The way кристаллизации same расплава of structure М proceeds up to temperature t3. At this temperature in balance there are three phases: liquid structure of a point n, laying on a boundary curve and two firm phases are crystals In and АС. The way кристаллизации from temperature t1 - t3 is defined(determined). The contents of liquid and all firm phases, total contents В+АС, and then contents In and АС separately at first is defined(determined):
The contents ж.ф. = =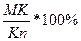
The contents АС+В = 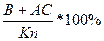
Содержаниие АС in тв.фазе = 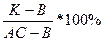
Содержаниие In in твф. = 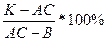
3. For definition of quantitative structure of a firm phase at the moment кристаллизации it is necessary at given temperature and structure расплава to lead(carry out) касательную to a point on a boundary curve, and to continue her(it) before crossing this direct connecting straight line. The point of crossing will divide(share) a connecting line into pieces of length, which will characterize a parity(ratio) two allocated thus кристаллизацию of two firm phases:
System Na2O—CaO—SiO2
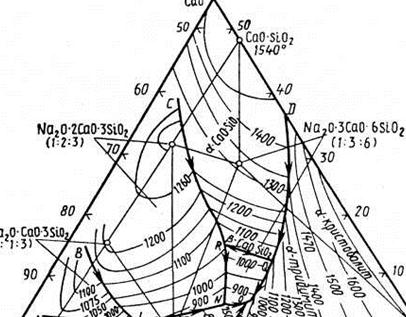
On fig. the diagramme of a condition of the investigated part of three-componental system Na2O-CaO-SiO2 on G.Moreju and N.Bouenu who in highbases areas is limited by connection Na2O-SiO2, and in highalumina - СаО-SiO2 is presented. In this part of system except already considered binary connections in private systems Na2O-SiO2 and CaO-SiO2 there are three threefold connections: Na2O-2CaO-3SiO2 (1:2:3) 2Na2O-CaO-3SiO2 (2:1:3) and Na2O-3CaO-6SiO2 (1:3:6) [in brackets the reduced designation of threefold connections accepted in this system is specified, at which on the first place the quantity of substance (moles) alkaline, on the second - shchelochno-zemelynogo oxide and on the third place - silica in the given connection is put. Except specified in system Na2O-SaO-SiO2 other threefold connections, in particular 2Na2O-8CaO-5SiO2 and 2Na2O-4CaO-3SiO2, (these structures, according to some information, represent firm solutions of alkaline silicates in orthosilicate of calcium), and also Na2O-CaO-SiO2, 4Na2O-3CaO-5SiO2, Na2O-2CaO-2SiO2 are marked also. Structures of these connections, fields of which primary crystallisation with sufficient degree of accuracy are not defined, lay outside of 69 parts of system presented on fig.
Rich of silica threefold connection d e v i t r i t Na2O-3CaO-6SiO2 melt incongruent at 1047°С, decaying on crystals β-CaO-SiO2 and alloy. Devitrit in the form of spherical crystal joints or thin needle or prism crystals often takes shape as one of phases at devitrification usual glasses. In the field of devitrit (in its top part) structures of the glasses most proof to action of water and alkaline solutions settle down.
Connection 2Na2O-CaO-3SiO2 also melt incongruent, decaying at 1140°С on crystals Na2O-2CaO-3SiO2 and a liquid. Connection Na2O-2CaO-3SiO2 melt at 1284°С without decomposition.
Structures in a considered part of system Na2O-SaO-S1O2 are characterised comparative easymelt. So, for example, all structures getting to elementary triangle Na2O-2SiO2-Na2O·3CaO·6SiO2-SiO2) begin melt at 725°С (eutectic structure About on the condition diagramme, fig.).
System Na2O-CaO-SiO2 has great value for the "know-how" alumina-sodium silicate glasses. It includes structures of some industrial glasses (window, bottle, tare and so forth) in which SiO2, Na2O and СаО are the main components.
As well as any other diagramme of a condition, system Na2O-SaO-SiO2 diagramme expresses only equilibrium conditions which, as is known, glass does not concern. Nevertheless, the knowledge of the diagramme of a condition of this system is necessary in glasses as with theoretical, and purely practical point of view. In manufacturing techniques of industrial glasses the knowledge of diagrammes of a condition of corresponding systems is necessary for struggle against one of rather widespread defects or as them sometimes name, defects of glass - stones of crystallization which represent crystal inclusions in the glass, breaking its physical and chemical uniformity. At crystallization silica-sodium silicate glasses the crystal phases existing in system Na2O-SaO-SiO2 are formed. In usual industrial glasses are formed most often trydimite, crystobalite, an vollastonit, , devitrit.
Principal cause of crystallization of glasses are incorrectly chosen structure inclined to crystallization and infringements of a temperature mode of cooking and glass development. Struggle against propensity of glasses to crystallization demands knowledge of the nature of a phase dropping out at crystallizations, temperature limits in which has flown down can crystallizations (in particular, temperatures of the beginning of crystallization), and speeds of crystallization. The condition diagramm allows not only to answer precisely at least on the two first question, but also to draw certain qualitative conclusions concerning speed of crystallization. It is known, in particular, that the glasses corresponding on structure to certain chemical compounds, have the greatest speed of crystallization. The structures forming at crystallization a firm phase, differing from structure of initial glass, will crystallize more slowly. Most difficultly with other things being equal crystallize eutectics structures.
System CaO-AI2O3-SiO2
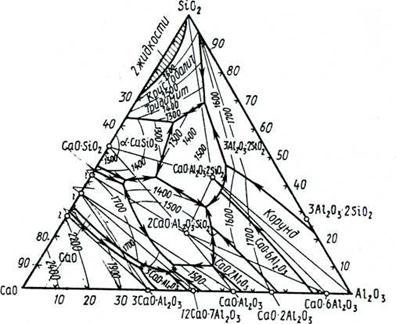
System SaO-Ai2O3-SiO2 has been in details investigated for the first time by G.Rankinym and F.Right. Further some specifications and changes have been brought in the diagramm of a condition of this system offered by them. The specified diagramm of a condition of system SaO-A12O3-SiO2, on E.Osbornu and M.Muanu, is presented on fig. In system SaO-Ai2O3-SiO2 there are many chemical compounds, including a little binary and two threefold.
Binary connections are presented by calcium silicates - 3CaO-SiO2, 2CaO-SiO2, 3CaO-2SiO2 and CaO-SiO2, alumosilicate 3Al2Os-2SiO2 which were considered earlier at the description of the double systems SaO-SiO2 and А12О3-SiO2, and calcium aluminates-3CaO -A12O3 (melted incongruent at 1535°С), 12СаО-7А12О3 (According to some information, this connection has the structure expressed by the formula 5SaO-Za12O3 (melted incongruent at 1455 °), СаО-А12О3 (melted incongruent at 1600°С), СаО-2А12О3 (earlier formula ЗСаО-5Аl2Оз) (melted incongruent was attributed at 1730oС, however there are data and about incongruent fusion this connection at 1765°С) and СаО-6А12О3 (melted incongruent but at 1850°С).
Threefold connections in this system are presented anortite (limy feldspar) CaO-Al2O3·SiO2 and gelenite 2CaO-Al2O3-SiO2. Both these connections melting without decomposition: the first at 1550 and the second at 1590°С. There are data about three-updating anortite - threeclinnic, rhombic and hexagonal, and hexagonal anortite is stable to 300°С at which it passes in threeclinnic anortite, stable up to fusion temperature (1550°С), and rhombic anortite metastable at all temperatures. On other data, the form anortite, and rhombic and hexagonal forms - metastable is stable only threeclinnic. Anortite widespread in the nature mainly in the form of continuous firm solutions with albite Na2O-Al2O3-6SiO2, named plagioklaz minerals.
Galenite has no polymorphic versions. This mineral meets in the nature usually in the form of unlimited firm solutions with okermanite 2CaO-MgO-2SiO2, named melilite.
It is necessary to notice, that in considered system at high pressures there are two more threefold connections - 3CaO-Al2O3-3SiO2 and piroxen structure CaO-Al2O3-SiO2 which at usual pressure have no on the diagramme of areas of stable existence and on the diagramm (fig.) are not presented.
System SaO-A12O3-SiO2 plays the important role in technology of reception portlandcement, glay cement, dinases , glasses, thin ceramics, in studying of processes of formation and properties of sour and basic domain slags and so forth On fig. the triangle of structures of this system on which the areas corresponding to structures applied in the technician of various technical products are allocated is presented.
System MgO—А12О3—SiO2
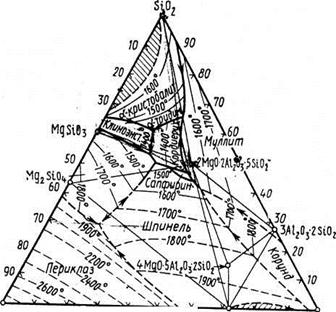 The modern kind of the three-componental diagramme of conditions MgO-А12О3-SiO2, the offered E.Osbornom and A.Muanom on the basis of generalisation of available data of research of this system, is presented on fig.
The modern kind of the three-componental diagramme of conditions MgO-А12О3-SiO2, the offered E.Osbornom and A.Muanom on the basis of generalisation of available data of research of this system, is presented on fig.
In system except already considered binary connections - magnesium silicates (2MgO-SiO2, MgO-SiO2) and aluminium (3Al2O3-2SiO2) there is one more binary connection-magnezial, or noble, MgO-Аl2Оз, of great importance in technology of ceramics. Shpinel melted congruent at 2135°С. In some works it has been established, what exactly shpinel is a primary product solidphase reactions in system MgO-A12O3-SiO2 at a various parity initial oxide, that speaks in the greatest speed of its formation.
Threefold connections in this system are presented cordierite and volume 2MgO-2Al2O3-5SiO2 and sapfirine 4MgO-5Al2O3-2SiO2.
Cordierite melted incongruent at 1540°С, decaying on a liquid and crystals of mullite. Cordierite differs difficult and up to the end not studied polymorphism, forming some polymorphic forms and intermediate phases, besides, for it formation of areas of uniformity (firm solutions) is characteristic. Sometimes all these versions name cordieritosimilar phases.
There are data about existence following cordieritosimilar phases.
High-temperature hexagonal α-cordierite (indialitic a phase - under the name structurally similar with artificial α-cordierite a natural mineral indialite, having with Becoming cordierite ). α-Cordierite represents a disorder phase, in which structure А13 +, replacing Si4 + in tetraedrical groups SiO44 - it is distributed statistically. α-Kordierit about
It will be undressed at high-temperature (1000... 1300°С) fast crystallisation of glasses of structure cordierite or close to it as metastable cordieritosimilar the phase as at heat treatment in a wide interval of temperatures through a series of intermediate connections turns in β-cordierite also is considered,
Lowtemperature rhombic β-cordierite - it is ordered and in a wide interval of temperatures stable, than α-cordierite, a version cordierite. It turns out lowtemperature (more low 950°С) long crystallization of glasses cordierite structure.
Osumullitic hexagonale the phase (has similarity to a mineral osumullite) represents cordieritosimilar the metastable phase formed at crystallization of glasses, with
Holding it is a little bit more silica, than in cordierite.
Petalitic phase (in the structural relation it is similar to a mineral petalite Li2O·Al2O3·8Si2O)- cordieritosimilar the metastable version formed at crystallization of glasses rich oxide magnesium and silicon.
5. μ-Cordierite - unstable cordieritosimilar a phase with variable structure from 2: 2: 5 to 1:1:3 (MgO: Al2O3:SiO2), similar on structure with Li2O·Al2O3·4Si2O.
Cordieritosimilar phases can exist in the form of firm solutions. According to some information, isomorphic replacements in cordierite can occur, for example, under following schemes:
Mg2 + + Si4 ++ = 2А13 + and 2А13 + + Mg2 + = 2 Si4 +
As a result of such replacements can arise cordieritosimilar firm solutions much either a lack silica or alumina. It is necessary to note propensity of connections of considered system to formation of firm solutions which, for example, have been found out in private systems 2MgO·2Al2O3·5SiO2-MgO·SiO2, MgO·А12О3-2MgO·SiO2, MgO-2MgO·SiO2 etc. Metastable quartzsimilar firm solutions with structure high-temperature α-quartz are found out between SiO2 and MgO-Al2O3.
Phase parities between various versions cordierite become complicated formation of intermediate phases with various degree of orderliness. Temperature areas of stable or metastable existence separate cordieritosimilar phases can change depending on their structure. N.A.Toropov has established, that hexagonal α-cordierite after long endurance at temperature 1400°С passes in rhombic β-cordierite, steady to 1440°С. At 1460°С there is a return transition β-cordierite in high-temperature α the-form. As this transition is reversible, it is possible to assume, that α-cordierite has at heats area of stable existence.
The second designated on the diagramm of a condition of system MgO-A12O3-SiO2 threefold connection is sapfirin 4MgO-5Al2O3-2SiO2 (other formulas of sapfirin with a little bit other parity oxide are offered also). This connection melted incongruent at 1475°С, decaying on a liquid and shpinel.
It is known and some more alumosilicate magnesium though any of them cannot exist at normal pressure stably in contact with alloy the given system. To such connections belongs, in particular, meeting in the nature пироп 3MgO-Al2O3-3SiO2, concerning group of pomegranates. Pirop has been synthesized at the raised pressure - (6-12) • 103 МПа and temperature 1500± 150°С.
System MgO-A12O3-SiO2 matters for technology of special pottery glasscrystallic materials and, in particular, has special value for reception ceramic and glasscrystallic materials with exclusively low and even in negative factor of thermal expansion on a basis cordierite .
Читайте також:
- BASIC NOTIONS OF THE LECTURE.
- BASIC NOTIONS OF THE LECTURE.
- LECTURE 1. Contrastive Stylistic as a Linguistic Discipline
- Lecture 12. Evolution of the ME Lexical System.
- LECTURE 13
- Lecture 14. Evolution of the ME Nominal Morphology.
- Lecture 16
- Lecture 16
- Lecture 4
- LECTURE EIGHT
- LECTURE ELEVEN
- LECTURE FIVE
| <== попередня сторінка | | | наступна сторінка ==> |
| | |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |