
РЕЗОЛЮЦІЯ: Громадського обговорення навчальної програми статевого виховання
ЧОМУ ФОНД ОЛЕНИ ПІНЧУК І МОЗ УКРАЇНИ ПРОПАГУЮТЬ "СЕКСУАЛЬНІ УРОКИ"
ЕКЗИСТЕНЦІЙНО-ПСИХОЛОГІЧНІ ОСНОВИ ПОРУШЕННЯ СТАТЕВОЇ ІДЕНТИЧНОСТІ ПІДЛІТКІВ
Батьківський, громадянський рух в Україні закликає МОН зупинити тотальну сексуалізацію дітей і підлітків
Відкрите звернення Міністру освіти й науки України - Гриневич Лілії Михайлівні
Представництво українського жіноцтва в ООН: низький рівень культури спілкування в соціальних мережах
Гендерна антидискримінаційна експертиза може зробити нас моральними рабами
ЛІВИЙ МАРКСИЗМ У НОВИХ ПІДРУЧНИКАХ ДЛЯ ШКОЛЯРІВ
ВІДКРИТА ЗАЯВА на підтримку позиції Ганни Турчинової та права кожної людини на свободу думки, світогляду та вираження поглядів
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Read the text, translate it and answer which sentences below are true and which are false.
Fusion
The Melting Point. - If a vessel of ice or snow is heated, the temperature at first rises until it is 0 °C and then remains stationary until all the ice is melted. After all the ice has been melted, the temperature of the water begins to rise. That temperature at which the solid changes into a liquid without a change of temperature is called the melting point. For ice this temperature is 0 °C or 32 °F. At the melting point, the addition of heat simply serves to hasten the melting process without any change of temperature.
If a pail of water is placed in a freezing mixture of ice and snow, the temperature of the water decreases until ice begins to be formed in the pail. After this temperature has been reached, the temperature of the water in the pail remains the same until all the water has become ice. That temperature at which the liquid changes into the solid state is its freezing point. This temperature is ordinarily the same as the temperature at which the solid melts. For crystalline substances, such as ice or copper, the freezing point or the melting point is sharply defined. For substances that are not crystalline, such as wax or glass, the substance gradually softens in passing from the solid to the liquid state. Such substances do not have a definite melting point. In the cases of certain fats, the melting point is not the same as the freezing point. For example, butter melts between 28 °С and 32 °С and solidifies between 20 °С and 23 °C.
Heat оf Fusion. - In order to cause a solid like ice to change into a liquid, it is necessary to supply a given quantity of heat to each gram or each pound of it. The heat of fusion of a substance is defined to be the number of calories necessary to convert 1 g at the melting point into liquid at the same temperature. It may also be defined as the number of Btu that must be supplied to change 1 lb of the solid to liquid without a change of temperature. To change 1 g of ice to water at 0 °C requires 80 cal, and to convert 1 lb of ice to water at 32 °F requires 144 Btu.
Effect of Pressure on the Melting Point. - Since an increase of pressure tends to cause a body to contract, the melting point of ice, which contracts on melting, is lowered by the application of pressure. Careful experiments show that this lowering is 0.0075 °С for an increase of 1 atm of pressure. If, on the other hand, a substance expands upon melting, its melting point will be raised by the application of pressure.
The effect of pressure on the melting point of ice may be shown by taking a piece of ice which is about 1.5 ft long and 6 in. square, and hanging over it a loop of wire from which a weight of 35 or 40 lb is supported. The pressure of the wire on the ice lowers the melting point of the ice, so that it is in a condition to melt as soon as the necessary heat is supplied. In order to melt each gram, it is necessary to supply 80 cal to it. This heat is taken from the water above the wire, causing it to freeze again. This process continues, until the wire cuts its way through the block of ice, leaving the block as solid as it was at the beginning of the experiment.
Boiling Point of Water.- Fill a flask half full of water, and insert a thermometer in one of the holes in the stopper. In the other hole insert a short glass tube through which the steam may escape. Heat the flask over a flame until the water boils. By reading the thermometer from time to time, it will be found that no matter how rapidly the heat is applied, the temperature does not rise above 100 °C. It will be noticed that at a certain temperature bubbles forming at the bottom of the flask rise to the surface, growing in size as they rise. That temperature at which the bubbles begin to reach the surface of any given liquid is called the boiling point or the boiling temperature. The boiling point can be defined as the temperature at which the pressure of the saturated vapor of the liquid is equal to the pressure of the atmosphere on the surface of the liquid.
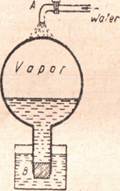
Effect of Pressure on the Boiling Point. - Since the boiling point of a liquid is the temperature at which the vapor pressure of the liquid is the same as the outside pressure on it, it follows at once that when the outside pressure is changed, the boiling point will also change. This is easily understood if we recall that ordinarily the pressure of the atmosphere is 14.7 lb to the square inch. If this pressure is decreased, it will not be necessary to raise the temperature so high in order to allow the bubbles to form. When the pressure is raised, it will be necessary to raise the temperature still higher in order to produce bubbles. The bubbles will form only when the pressure of the vapor in the bubble is equal to the pressure on the surface of the liquid.
| <== попередня сторінка | | | наступна сторінка ==> |
| Figure 32 - One British thermal unit is the | | | Figure 33 - The boiling point is lowered by |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |