
РЕЗОЛЮЦІЯ: Громадського обговорення навчальної програми статевого виховання
ЧОМУ ФОНД ОЛЕНИ ПІНЧУК І МОЗ УКРАЇНИ ПРОПАГУЮТЬ "СЕКСУАЛЬНІ УРОКИ"
ЕКЗИСТЕНЦІЙНО-ПСИХОЛОГІЧНІ ОСНОВИ ПОРУШЕННЯ СТАТЕВОЇ ІДЕНТИЧНОСТІ ПІДЛІТКІВ
Батьківський, громадянський рух в Україні закликає МОН зупинити тотальну сексуалізацію дітей і підлітків
Відкрите звернення Міністру освіти й науки України - Гриневич Лілії Михайлівні
Представництво українського жіноцтва в ООН: низький рівень культури спілкування в соціальних мережах
Гендерна антидискримінаційна експертиза може зробити нас моральними рабами
ЛІВИЙ МАРКСИЗМ У НОВИХ ПІДРУЧНИКАХ ДЛЯ ШКОЛЯРІВ
ВІДКРИТА ЗАЯВА на підтримку позиції Ганни Турчинової та права кожної людини на свободу думки, світогляду та вираження поглядів
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Heat and Work
Transformation of Heat into Work. -The heat engines that play such a large part in modern life depend on the transformation of heat into work. The heated steam in the cylinder of a steam engine does work in pushing the piston back. This work is available for driving the machinery connected to the engine. A gasoline engine can drive an automobile or a tractor only when it is supplied constantly with heat from the exploding gasoline in the cylinders. In these cases, heat is transformed into work.
First Law of Thermodynamics. - The first law of thermodynamics is a special case of the law of conservation of energy. It is implied in the definition of the mechanical equivalent of heat and may be expressed by the equation
W=JH,
where W = the work measured in work units;
H = heat measured in heat units;
J = the mechanical equivalent of heat.
More specifically, the law states that when any mechanical change occurs in an isolated system, the energy of the system remains constant. Heat may be transformed into work or work into heat, but the total energy of the system remains unchanged. In other words, the first law of thermodynamics states that in the transformation of work into other forms of energy or in the transformation of one form of energy into other forms of energy, no energy is ever created or destroyed. The energy before and after the transformation is always the same. This law in its general form can not be proved by experiment but conclusions based on it have always been confirmed by experiment.
Second Law of Thermodynamics. - The second law of thermodynamics states the conditions under which heat may be transferred from one body to another. It is in effect a statement of the fact that heat naturally flows from a place of higher to one of lower temperature but never in the reverse direction. An analogue may make the meaning clearer. Water may flow from a higher to a lower level with the performance of work. Heat may flow from a higher to a lower temperature with the performance of work. To make water flow from a lower to a higher level requires external work to be done on it. To cause heat to flow from a lower to a higher temperature also requires the performance of external work. The natural tendency of heat to flow from a higher to a lower temperature makes it possible for a heat engine to transform heat into work. On the contrary, a mechanical refrigerating machine must transfer heat from a colder to a hotter body. Work must be done on such a machine to make this transfer. The following is one form of statement of the law:
It is impossible for any kind of a machine working in a cycle to transfer heat from a lower to a higher temperature unless external work is done on it. A similar statement of the water analogy would be: It is impossible for a pump working in a cycle to transfer water from a lower to a higher level unless external work is done on it. The law cannot be proved by direct experiment. It is a generalization based on the fact that in all human experience no contradictionsof the law have been found. It merely states that heat of itself can flow only from higher to lower temperatures and no exceptions to this rule are known.
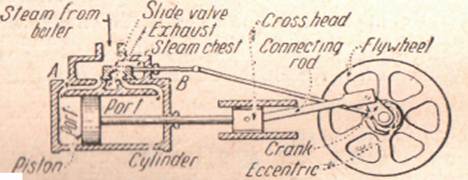
Steam Engine. — In a steam engine (Figure 34) a closely fitting piston moves in a cylinder that is connected to the steam chest by means of two pipes, which are provided with valves, serving alternately as inlet and exhaust for the steam. As the piston moves forward, steam enters through A and the used steam is forced out through B.
| <== попередня сторінка | | | наступна сторінка ==> |
| Transfer of Heat | | |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |