
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Scintillation neutron detectors
Scintillation neutron detectors include liquid organic scintillators,[6] crystals,[7][8] plastics, glass[9] and scintillation fibers.[10]
60. Fundamental forces. There are 4 fundamental forces that have been identified. In our present Universe they have rather different properties. Properties of the Fundamental Forces
· The strong interaction is very strong, but very short-ranged. It acts only over ranges of order 10-13 centimeters and is responsible for holding the nuclei of atoms together. It is basically attractive, but can be effectively repulsive in some circumstances.
· The electromagnetic force causes electric and magnetic effects such as the repulsion between like electrical charges or the interaction of bar magnets. It is long-ranged, but much weaker than the strong force. It can be attractive or repulsive, and acts only between pieces of matter carrying electrical charge.
· The weak force is responsible for radioactive decay and neutrino interactions. It has a very short range and, as its name indicates, it is very weak.
· The gravitational force is weak, but very long ranged. Furthermore, it is always attractive, and acts between any two pieces of matter in the Universe since mass is its source. The Tortoise and the Hare: Gravity Always Wins. The four fundamental forces all play central roles in making the Universe what it is today, but with respect to the large-scale issues that are of interest to cosmology it is gravitation that is most important. This is because of two of its basic properties that set it apart from the other forces: (1) it is long-ranged and thus can act over cosmological distances, and (2) it always supplies an attractive force between any two pieces of matter in the Universe. Thus, although gravitation is extremely weak, it always wins over cosmological distances and therefore is the most important force for the understanding of the large scale structure and evolution of the Universe. Unification of the Forces of Nature. Although the above discussion indicates that the fundamental forces in our present Universe are distinct and have very different characteristics, the current thinking in theoretical physics is that this was not always so. There is a rather strong belief (although it is yet to be confirmed experimentally) that in the very early Universe when temperatures were very high compared with today, the weak, electromagnetic, and strong forces were unified into a single force. Only when the temperature dropped did these forces separate from each other, with the strong force separating first and then at a still lower temperature the electromagnetic and weak forces separating to leave us with the 4 distinct forces that we see in our present Universe. The process of the forces separating from each other is called spontaneous symmetry breaking.
8. Ionization losses. The energetic particle is usually decelerated, it suffers energy losses called Coulomb losses or ionization losses depending on the interaction partner, usually an electron, being free or bound. The particle will also (slowly) change direction, so we are dealing with scattering. Finally, the energy loss and the scattering are the results of acceleration, to which are charges react by radiating. This radiation process is called bremsstrahlung or free-free radiation.
35. The creation of electron-positron pair. Pair production refers to the creation of an elementary particle and its antiparticle, usually when a photon (or another neutral boson) interacts with a nucleus or another boson. For example an electron and its antiparticle, the positron, may be created. This is allowed, provided there is enough energyavailable to create the pair – at least the total rest mass energy of the two particles – and that the situation allows both energy and momentum to be conserved. Other pairs produced could be a muon and anti-muon or a tau and anti-tau. However all other conserved quantum numbers (angular momentum, electric charge, lepton number) of the produced particles must sum to zero – thus the created particles shall have opposite values of each other. For instance, if one particle has electric charge of +1 the other must have electric charge of −1, or if one particle has strangeness of +1 then another one must have strangeness of −1. The probability of pair production in photon-matter interactions increases with increasing photon energy and also increases with atomic number approximately as Z2. γ + γ → e− + e+
In nuclear physics, this occurs when a high-energy photon interacts with a nucleus. The energy of this photon can be converted into mass through Einstein’s equation, E=mc2; where E is energy,m is mass and c is the speed of light. The photon must have enough energy to create the mass of an electron plus a positron. The rest mass of an electron is 9.11 × 10−31 kg (0.511 MeV), the same as a positron. Without a nucleus to absorb momentum, a photon decaying into electron-positron pair (or other pairs for that matter) can never conserve energy and momentum simultaneously
There are different processes how an electron-positron pair can be produced. In air (e.g. in lightning discharges) the most important one is the scattering of photons at the nuclei of atoms or molecules. Quantum mechanically, the process of pair production can be described by the quadruply differential cross section:[2]
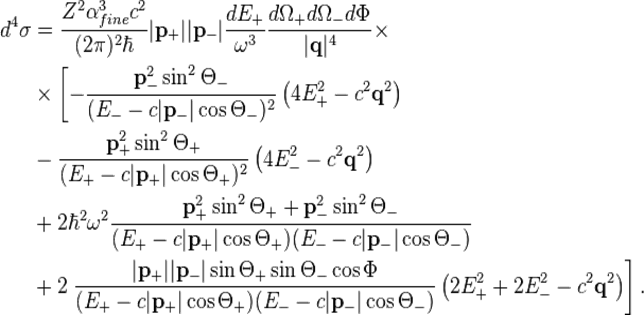
With 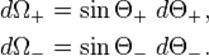
This expression can be derived by using a quantum mechanical symmetry between pair production and Bremsstrahlung.
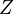 is the atomic number,
is the atomic number,  the fine structure constant,
the fine structure constant, 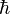 the reduced Planck's constant and
the reduced Planck's constant and  the speed of light. The kinetic energies
the speed of light. The kinetic energies 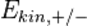 of the positron and electron relate to their total energies
of the positron and electron relate to their total energies 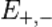 and momenta
and momenta 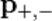 via
via
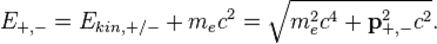
Conservation of energy yields
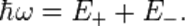
The momentum 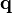 of the virtual photon between incident photon and nucleus is:
of the virtual photon between incident photon and nucleus is:
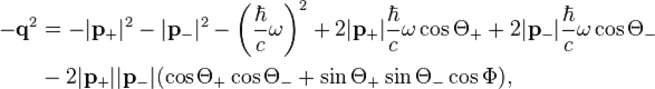
where the directions are given via:

where 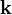 is the momentum of the incident photon.
is the momentum of the incident photon.
In order to analyse the relation between the photon energy 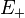 and the emission angle
and the emission angle 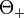 between photon and positron, Köhn and Ebert integrated [3] the quadruply differential cross section over
between photon and positron, Köhn and Ebert integrated [3] the quadruply differential cross section over 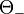 and
and 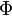 . The double differential cross section is:
. The double differential cross section is:
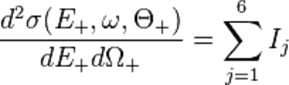
44. Units of radiation dose. Radiation absorbed dose and effective dose in the international system of units (SI system) for radiation measurement uses "gray" (Gy) and "sievert" (Sv), respectively. In the United States, radiation absorbed dose, effective dose, and exposure are sometimes measured and stated in units called rad, rem, or roentgen (R). For practical purposes with gamma and x rays, these units of measure for exposure or dose are considered equal.
This exposure can be from an external source irradiating the whole body, an extremity, or other organ or tissue resulting in an external radiation dose. Alternately, internally deposited radioactive material may cause an internal radiation dose to the whole body, an organ, or a tissue.
Smaller fractions of these measured quantities often have a prefix, such as milli (m) that means 1/1,000. For example, 1 sievert = 1,000 mSv. Micro (μ) means 1/1,000,000. So, 1,000,000 μSv = 1 Sv, or 10 μSv = 0.000010 Sv. Conversions from the SI units to older units are as follows:
· 1 Gy = 100 rad
· 1 mGy = 100 mrad
· 1 Sv = 100 rem
· 1 mSv = 100 mrem
With radiation counting systems, radioactive transformation events can be measured in units of "disintegrations per second" (dps) and, because instruments are not 100 percent efficient, "counts per second" (cps). Gray (unit)
The gray (symbol: Gy) is the SI derived unit of absorbed dose, specific energy (imparted) and of kerma. Such energies are typically associated with ionizing radiation such as X-rays or gamma particles or with other nuclear particles. It is defined as the absorption of one joule of such energy by one kilogram of matter. Unlike the pre-1971 roentgen, the gray has always been defined independently for any target material. The same beam of 1 roentgen would impart more grays to biological tissue than it does to air. The gray is sometimes used to measure beam kerma, in which case the reference target material must be defined explicitly. (Usually dry air at standard temperature and pressure.) The gray was named after the British physicist Louis Harold Gray, a pioneer in the field of measurement of radium radiation and X-rays and their effects on living tissue,[2] and was adopted as part of SI by the 15th CGPM in 1975. The SI unit is similar to the traditional cgs unit, the rad (equivalent to 0.01 Gy), which remains common in industry in the United States, while "strongly discouraged" in the style guide for U.S. National Institute of Standards and Technology authors. One gray is the absorption of one joule of energy, in the form of ionizing radiation, per kilogram of matter.
 The sievert (symbol: Sv) is the International System of Units' (SI) derived unit of equivalent radiation dose, effective dose, and committed dose. Quantities that are measured in sieverts represent the stochastic biological effects of ionizing radiation.
The sievert (symbol: Sv) is the International System of Units' (SI) derived unit of equivalent radiation dose, effective dose, and committed dose. Quantities that are measured in sieverts represent the stochastic biological effects of ionizing radiation.
The sievert represents a measure of the biological effect, and should not be used to express the unmodified absorbed dose of radiation energy, which is a physical quantity measured in grays. To enable consideration of biological effects, further calculations must be performed to convert absorbed dose into equivalent and effective dose, the details of which depend on the radiation type and biological context. This can be a complex calculation, so for applications such as radiation protection and dosimetry assessment the International Committee on Radiation Protection has published weighting factors which can be used to calculate the biological effects on the human body.
The sievert is of fundamental importance in radiation dosimetry, and is named after Rolf Maximilian Sievert, a Swedish medical physicist renowned for work on radiation dosage measurement and research into the biological effects of radiation. One sievert equals 100 rem, an older unit of measurement still in widespread use. One sievert carries with it a 5.5% chance of eventually developing cancer.[1] Doses greater than 1 sievert received over a short time period are likely to cause radiation poisoning, possibly leading to death within weeks.
To enable a comprehensive view of the sievert, this article deals with the definition of the Sievert as an SI unit, the recommendations of the ICRP on how the sievert is calculated and a guide to the effects of ionizing radiation as measured in sieverts.
53. Dosimetric controlling npp.A nuclear power plant is a thermal power station in which the heat source is a nuclear reactor. As is typical in all conventional thermalpower stations the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity. As of 16 January 2013, the IAEA report there are 439 nuclear power reactors in operation operating in 31 countries. Nuclear power plants are usually considered to be base load stations, since fuel is a small part of the cost of production. Radiation safety of personnel at nuclear power plants (NPP) is a priorit y aim. Degree of radiation exposure of personnel is defined by many factors: NPP design, operation of equipment, organizational management of radiation hazardous works and, certainly, safety culture of every employee. Automated Personal Dosimetry Monitoring System (APDMS) is applied at all nuclear power plants nowadays in Russia to eliminate the possibility of occupational radiation exposure beyond regulated level under different modes of NPP operation. APDMS provides individual radiation dose registration. In the paper the efforts of Design Bureau “Promengineering” in construction of software and hardware complex of APDMS (SHW APDMS) for NPP with PWR are presented. The developed complex is intended to automatize activities of radiation safety department when caring out individual dosimetry control. The complex covers all main processes concerning individual monitoring of external and internal radiation exposure as well as dose recording, management, and planning. SHW APDMS is a multi-purpose system which software was designed on the modular approach. This approach presumes modification and extension of software using new components (modules) without changes in other components. Such structure makes the system flexible and allows modifying it in case of implementation a new radiation safety requirements and extending the scope of dosimetry monitoring. That gives the possibility to include with time new kinds of dosimetry control for Russian NPP in compliance with IAEA recommendations, for instance, control of the equivalent dose rate to the skin and the equivalent dose rate to the lens of the eye. SHW APDMS provides dosimetry control as follows: · Current monitoring of external radiation exposure:
- Gamma radiation dose measurement using
radiophotoluminescent personal dosimeters.
- Neutron radiation dose measurement using
thermoluminescent personal dosimeters (albedo dosimeters).
· Operational and emergency monitoring of external radiation exposure:
- Gamma radiation dose and dose rate measurement using
direct reading personal dosimeters.
- Gamma radiation dose measurement using
radiophotoluminescent personal dosimeters.
· Monitoring of internal radiation exposure:
- Measurement of activity of incorporated radionuclides using whole body counters.
Hardware of APDMS represents a complex of automated workplaces based on industrial computers and measuring equipment; all workplaces are connected to one local computational network. Client software installed on automated workplaces processes the results of dosimetry monitoring (spectrum processing, computation of personal dose, report generation, etc.) and provides data exchange with data base of APDMS in the remote server. Communication with APDMS server is organized via the local computational network Ethernet.
40. The radioactive capture. Electron capture a type of radioactive decay of nuclei in which a nucleus captures an electron from one of the inner shells of the atom, such as the K-, L-, or M-shell, and at the same time emits a neutrino. When this occurs, a nucleus with mass number A and atomic number Z is transformed into a nucleus with the same A and a Z that is smaller by 1: AZ + e– → AZ–1 + v. The vacancy formed in the atom’s electron shell is filled by electrons from other shells, and, as a result, a quantum of the characteristic X-radiation of the atom AZ–1 or a corresponding electron (an Auger electron) is emitted.
Electron capture is possible if the mass (in energy units) of the atom AZ greater than the mass of the atom AZ–1, by an amount greater than the binding energy of the captured electron. If this is greater than 2mc2 = 1.02 mega electron volts (where m is the rest mass of the electron and c is the speed of light), β+ decay begins competing with electron capture.
The nuclear capture of electrons (K capture) occurs by a process quite different from atomic capture and is in fact a consequence of the general beta interaction. This general interaction includes ß - decay (the oldest known beta transformation and hence the name), ß + decay (or positron decay), and K capture, the latter so called because the electron captured by the nucleus is taken from the K shell (the shell nearest the nucleus) of atomic electrons. A second-order process, called L capture, can also occur, in which (to speak pictorially and thus somewhat imprecisely) an s electron (from the K shell) is captured with the simultaneous transition of a p electron (from the L shell) to the K shell with the emission of gamma radiation.
49. Calorimetric method.Calorimetric measurements permit the direct determination of only the sum of the heats of the process under investigation and of various secondary processes, such as mixing, evaporation of water, and fracture of the ampul with material. The heat of the secondary processes must be determined experimentally or by calculation and must be omitted from the final result. One of the inevitable secondary processes is the heat exchange between the calorimeter and the surrounding medium through radiation and thermal conductivity. In order to take the secondary processes into account, primarily the heat exchange, the calorimetric system is surrounded by a jacket, the temperature of which is regulated.
The jacket temperature in liquid isothermal calorimeters is maintained at a constant level. The greatest difficulty in determining heats of chemical reactions is not due to problems related to consideration of secondary processes but to problems related to the determination of the completeness of the reaction and the necessity of taking several reactions into account.
In integrating calorimeters of another type, isothermal (constant temperature) calorimeters, the introduced heat does not change the temperature of the calorimetric system but causes changes in the aggregate state of a material that constitutes part of this system (for example, melting of ice in the Bunsen ice calorimeter). The quantity of introduced heat is calculated in this case from the mass of the material that has changed its state of aggregation (for example, the mass of melted ice, which can be measured from the change in volume of the ice-water mixture) and from the heat of phase transition
Aneroid integrating calorimeters are most frequently used for determining the enthalpy of materials at high temperatures (up to 2500°C). The calorimetric system in calorimeters of this type consists of a block of metal (usually copper or aluminum) with wells for the reaction vessel, the thermometer, and the heater.
58. Calculation of neutron radiation protection.Neutron radiation protection relies on radiation shielding. Due to the high kinetic energy of neutrons, this radiation is considered to be the most severe and dangerous radiation to the whole body when exposed to external radiation sources. In comparison to conventional ionizing radiation based on photons or charged particles, neutrons are repeatedly bounced and slowed (absorbed) by light nuclei, so hydrogen-rich material is more effective than iron nuclei. The light atoms serve to slow down the neutrons by elastic scattering, so they can then be absorbed by nuclear reactions. However, gamma radiation is often produced in such reactions, so additional shielding has to be provided to absorb it. Care must be taken to avoid using nuclei which undergo fission or neutron capture that results in radioactive decay of nuclei that produce gamma rays.
Neutron transport is the study of the motions and interactions of neutrons with materials. Nuclear scientists and engineers often need to know where neutrons are in an apparatus, what direction they are going, and how quickly they are moving. It is commonly used to determine the behavior of nuclear reactor cores and experimental or industrial neutron beams. Neutron transport is a type of radiative transport. The neutron transport equation is a balance statement that conserves neutrons. Each term represents a gain or a loss of a neutron, and the balance, in essence, claims that neutrons gained equals neutrons lost. It is formulated as follows:
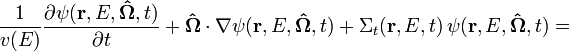
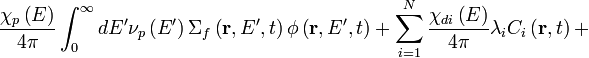
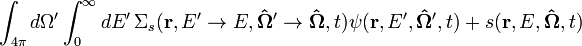
Several basic types of neutron transport problems exist, depending on the type of problem being solved.
4. The radioactivity law. The law of radioactive decay predicts how the number of the not decayed nuclei of a given radioactive substance decreases in the course of time. The red circles of this simulation symbolize 1000 atomic nuclei of a radioactive substance whose half-life period (T) amounts to 20 seconds. The diagram in the lower part of the applet represents the fraction of the not yet decayed nuclei (N/N0) at a given time t, predicted by the following law:
| N = N0 · 2−t/T |
N .... number of the not decayed nuclei
N0 ... number of the initially existing nuclei
t .... time
T .... half-life period
As soon as the applet is started with the yellow button, the atomic nuclei will begin to "decay" (change of color from red to black). You can stop and continue the simulation by using the button "Pause / Resume". In this case a blue point for the time and the fraction of the not yet decayed nuclei is drawn into the diagram. (Note that often these points don't lie exactly on the curve!) If you want to restore the initial state, you will have to click on the "Reset" button.
It is possible to give the probability that a single atomic nucleus will "survive" during a given interval. This probability amounts to 50 % for one half-life period. In an interval twice as long (2 T) the nucleus survives only with a 25 % probability (half of 50 %), in an interval of three half-life periods (3 T) only with 12,5 % (half of 25 %) and so on.You can't, however, predict the time at which a given atomic nucleus will decay. For example, even if the probability of a decay within the next second is 99 %, it is nevertheless possible (but improbable) that the nucleus decays after millions of years.
Radiation sickness. Radiation sickness is damage to your body caused by a large dose of radiation often received over a short period of time (acute). The amount of radiation absorbed by the body — the absorbed dose — determines how sick you'll be. Radiation sickness is also called acute radiation sickness, acute radiation syndrome or radiation poisoning. Common exposures to low-dose radiation, such as X-ray or CT examinations, don't cause radiation sickness.
Although radiation sickness is serious and often fatal, it's rare. Since the atomic bombings of Hiroshima and Nagasaki, Japan, during World War II, most cases of radiation sickness have occurred after nuclear industrial accidents such as the 1986 fire that damaged the nuclear power plant at Chernobyl or the 2011 earthquake that damaged the nuclear power plant on the east coast of Japan.
The absorbed dose of radiation is measured in a unit called a gray (Gy). Diagnostic tests that use radiation, such as an X-ray, result in a small dose of radiation — typically well below 0.1 Gy, focused on a few organs or small amount of tissue.
Signs and symptoms of radiation sickness usually appear when the entire body receives an absorbed dose of at least 1 Gy. Doses greater than 6 Gy to the whole body are generally not treatable and usually lead to death within two days to two weeks, depending on the dose and duration of the exposure.
12. Ionizing losses, Radiation losses of electrons. Any charged particle, electron or ion, suffers an electrostatic interaction when it passes through the Coulomb field of another charged particle.. The energetic particle is usually decelerated, it suffers energy losses called Coulomb losses or ionization losses depending on the interaction partner, usually an electron, being free or bound. The particle will also (slowly) change direction, so we are dealing with scattering. Finally, the energy loss and the scattering are the results of acceleration, to which are charges react by radiating. This radiation process is called bremsstrahlung or free-free radiation.
In physics, the radiation length is a characteristic of a material, related to the energy loss of high energy, electromagnetic-interacting particles with it.
High-energy electrons (>~10 MeV) predominantly lose energy in matter by bremsstrahlung, and high-energy photons by e+e− pair production. The characteristic amount of matter traversed for these related interactions is called the radiation length X0, usually measured in g·cm−2. It is both the mean distance over which a high-energy electron loses all but 1⁄e of its energy by bremsstrahlung, and 7⁄9 of the mean free path for pair production by a high-energy photon. It is also the appropriate scale length for describing high-energy electromagnetic cascades.
The radiation length for a given material consisting of a single type of nuclei can be approximated by the following expression:[1]
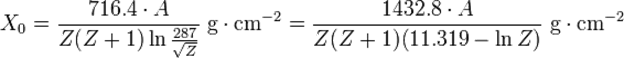 ,
,
where Z is the atomic number and A is mass number of the nucleus.
For electrons at lower energies (below few tens of MeVs), the energy loss by ionization is predominant.
While this definition may also be used for other electromagnetic interacting particles beyond leptons and photons, the presence of the stronger hadronic and nuclear interaction makes it a far less interesting characterisation of the material; the nuclear collision length and nuclear interaction length are more relevant.
39. Recoil nuclei may be illustrated by the behave our of a rifle. If it is held loosely during firing, its recoil, or “kick,” will be violent. If it is firmly held against the marksman’s shoulder, the recoil will be greatly reduced. The difference in the two situations results from the fact that momentum (the product of mass and velocity) is conserved: the momentum of the system that fires a projectile must be opposite and equal to that of the projectile. By supporting the rifle firmly, the marksman includes his body, with its much greater mass, as part of the firing system and the backward velocity of the system is correspondingly reduced. An atomic nucleus is subject to the same law. When radiation is emitted in the form of a gamma ray, the atom with its nucleus experiences a recoil due to the momentum of the gamma ray. A similar recoil occurs during absorption of radiation by a nucleus.
Finally, it is necessary to understand the principles governing the absorption of gamma rays by nuclei. Nuclei can exist only in certain definite energy states. For a gamma ray to be absorbed its energy must be exactly equal to the difference between two of these states. Such an absorption is called resonance absorption. A gamma ray that is ejected from a nucleus in a free atom cannot be resonantly absorbed by a similar nucleus in another atom because its energy is less than the resonance energy by an amount equal to the kinetic energy given to the recoiling source nucleus.
48. Photographic method.The photographic method used by Professor Powell is based on the fact that after an electrically charged particle has passed through a photographic emulsion, the silver bromide grains of the emulsion can be developed, making the path of the particle appear as a dark line which is, actually, a series of blackened grains with longer or shorter intervals between. The distance between the grains is proportional to the speed of the particle; the greater the speed of the particle, the greater the distance, which circumstance is connected with the fact that a swift particle has less power of ionizing than a slow one. The method is not new; it came into use in the early years of the 20th century as a means of demonstrating radioactive radiation. For the use of the method in the study of nuclear processes it was first necessary to have emulsions sensitive to various kinds of charged particles, and especially to very swift particles. The problem was brought nearer its solution in the early thirties when it was found that sensitizing the plates made them react to swift protons. The method was difficult, however, and it was not widely used.
In nuclear physics the photographic method had not been generally accepted even by the end of the thirties, despite the fact that various researchers had used it for studying cosmic radiation. Nuclear physicists were sceptical of the method because divergent results had been obtained in calculating the energy of the particles from measurements of the length of their traces. They placed more confidence in the so-called "Wilson chamber", where the radiation falls into an expansion chamber filled with moisture-saturated air or another gas. The gas is cooled by suddenly expanding the chamber, and drops of mist are deposited on the ions formed in the path of the particles. Under proper lighting, the paths of the particles which are in the chamber at the moment of expansion appear as cloud tracks.
5. The activity of radionuclides. A radionuclide, or a radioactive nuclide, is an atom with an unstable nucleus, characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or via internal conversion. During this process, the radionuclide is said to undergo radioactive decay, resulting in the emission of gamma ray(s) and/or subatomic particles such as alpha or beta particles. These emissions constitute ionizing radiation. Radionuclides occur naturally, or can be produced artificially.
Radionuclides are often referred to by chemists and physicists as radioactive isotopes or radioisotopes. Radioisotopes with suitable half-lives play an important part in a number of technologies (for example, nuclear medicine). Radionuclides can also present both real and perceived dangers to health. The number of radionuclides is uncertain because the number of very short-lived radionuclides that have yet to be characterized is extremely large and potentially unquantifiable. Even the number of long-lived radionuclides is uncertain (to a lesser degree), because many "stable" nuclides are calculated to have half-lives so long that their decay has not been experimentally measured. Radionuclides are used in two major ways: for their chemical properties and as sources of radiation. Radionuclides of familiar elements such as carbon can serve as radioactive tracers because they are chemically very similar to the nonradioactive nuclides, so most chemical, biological, and ecological processes treat them in a nearly identical way.
14. Characteristic X-ray radiation.Characteristic X-rays are emitted when outer-shell electrons fill a vacancy in the inner shell of an atom, releasing X-rays in a pattern that is "characteristic" to each element. Characteristic X-rays were discovered by Charles Glover Barkla in 1909, who later won the Nobel Prize in Physics for his discovery in 1917.
Characteristic X-rays are produced when an element is bombarded with high-energy electrons. When a high-energy electron (the incident electron) strikes a bound electron (the target electron) in an atom, the target electron is ejected from the inner shell of the atom. After the electron has been ejected, the atom is left with a vacant energy level, also known as a core hole. Outer-shell electrons then fall into the inner shell, emitting quantized photons with an energy level equivalent to the energy difference between the higher and lower states. Each element has a unique set of energy levels, and thus the transition from higher to lower energy levels produces X-rays with frequencies that are characteristic to each element. When an electron falls from the L shell to the K shell, the X-ray emitted is called a K-alpha X-ray. Similarly, when an electron falls from the M shell to the K shell, the X-ray emitted is called a K-beta X-ray. Sometimes, however, instead of releasing the energy in the form of an X-ray, the energy can be transferred to another electron, which is then ejected from the atom. This is known as the Auger effect, and the second ejected electron is known as an Auger electron. Characteristic X-rays can be used to identify the particular element from which they are emitted. This property is used in various techniques, including X-ray fluorescence spectroscopy, energy-dispersive X-ray spectroscopy, and wavelength-dispersive X-ray spectroscopy.
32. Photoelectric absorption. In a photoelectric absorption interaction, an incoming gamma-ray transfers virtually all of its energy to an atomic electron, usually the most tightly bound (K-shell) electron of an atom.
The atomic electron, now termed a photoelectron, is ejected from the atom with an energy equal to that of the initial gamma-ray minus the binding energy for the atomic electron:
Ee = Eg - Eb
The ejected electron is detected as a full energy peak in an energy spectrum. Photoelectric absorption facilitates the measurement of the energy of a gamma-ray photon. This interaction can also lead to the creation of x-ray fluorescence.
41. Nuclear reaction with neutron.Reactions with neutrons are important in nuclear reactors and nuclear weapons. While the best known neutron reactions are neutron scattering(the scattering of free neutrons by matter, can refer to either the physical process or the experimental technique which uses this process for the investigation of materials), neutron capture(a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus), and nuclear fission(is either a nuclear reaction or a radioactive decay process in which the nucleus of a particle splits into smaller parts), for some light nuclei (especially odd-odd nuclei) the most probable reaction with a thermal neutron is a transfer reaction.
Some reactions are only possible with fast neutrons:
· (n,2n) reactions produce small amounts of protactinium-231 and uranium-232 in the thorium cycle which is otherwise relatively free of highly radioactive actinide products.
· 9Be + n → 2α + 2n can contribute some additional neutrons in the beryllium neutron reflector of a nuclear weapon.
· 7Li + n → T + α + n unexpectedly contributed additional yield.
50. Chemical method (Colorimetry). In physical and analytical chemistry, colorimetry or colourimetry is a technique "used to determine the concentration of colored compounds in solution." A colorimeter is a device used to test the concentration of a solution by measuring its absorbance of a specific wavelength of light (not to be confused with the tristimulus colorimeter used to measure colors in general).
Colorimetry is "the science and technology used to quantify and describe physically the human color perception." It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color perception.
The absolute spectral power distribution of a light source can be measured with a spectroradiometer, which works by optically collecting the light, then passing it through a monochromator before reading it in narrow bands of wavelength.
Reflected color can be measured using a spectrophotometer (also called spectroreflectometer or reflectometer), which takes measurements in the visible region (and a little beyond) of a given color sample. If the custom of taking readings at 10 nanometer increments is followed, the visible light range of 400-700 nm will yield 31 readings. These readings are typically used to draw the sample's spectral reflectance curve (how much it reflects, as a function of wavelength)—the most accurate data that can be provided regarding its characteristics.
59. The cross section of neutrons, protons. The cross section is the probability that an interaction will occur between a projectile particle-say, a proton that has been accelerated-and a target particle, which could be an antiproton, or perhaps a proton or neutron in a piece of metal foil.
In nuclear and particle physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2.
The neutron cross section, and therefore the probability of an interaction, depends on:
· the target type (hydrogen, uranium…),
· the type of nuclear reaction (scattering, fission…).
· the incident particle energy, also called speed or temperature (thermal, fast…),
and, to a lesser extent, of:
· its relative angle between the incident neutron and the target nuclide,
· the target nuclide temperature.
7. Protons, alpha particles and heavy ions.The proton is a subatomic particle with the symbol p or p+ and a positive electric charge of 1 elementary charge. The free proton is a stable particle that has not been observed to break down spontaneously to other particles. In chemistry, the term proton refers to the hydrogen ion, H+.Since the atomic number of hydrogen is 1, a hydrogen ion has no electrons and corresponds to a bare nucleus, consisting of a proton. The free proton, thus, has an extremely short lifetime in chemical systems such as liquids and it reacts immediately with the electron cloud of any available molecule. In aqueous solution, it forms thehydronium ion, H3O+, which in turn is further solvated by water molecules in clusters such as [H5O2]+ and [H9O4]+. The transfer of H+ in an acid–base reaction is usually referred to as "proton transfer". The acid is referred to as a proton donor and the base as a proton acceptor. Likewise, biochemical terms such as proton pump and proton channel refer to the movement of hydrated H+ ions. The ion produced by removing the electron from a deuterium atom is known as a deuteron, not a proton. Likewise, removing an electron from a tritium atom produces a triton. The nucleus of a heavy element. When such nuclei are caused to collide at high velocities, new elements are created.Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. The symbol for the alpha particle is α or α2+. Because they are identical to helium nuclei, they are also sometimes written as He2+ or 4 2He2+ indicating a Helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle can be written as a normal (electrically neutral) Helium atom 42He.The nucleus of a heavy element. When such nuclei are caused to collide at high velocities, new elements are created.
34. Compton effect. Compton scattering is an inelastic scattering of a photon by a free charged particle, usually an electron. It results in a decrease in energy (increase in wavelength) of the photon (which may be an X-ray or gamma ray photon), called the Compton effect. Part of the energy of the photon is transferred to the scattering electron. Inverse Compton scattering also exists, in which a charged particle transfers part of its energy to a photon. Compton scattering is an example of inelastic scattering, because the wavelength of the scattered light is different from the incident radiation. Still, the origin of the effect can be considered as an elastic collision between a photon and an electron. The amount the wavelength changes by is called the Compton shift. Compton scattering usually refers to the interaction involving only the electrons of an atom. The Compton effect was observed by Arthur Holly Compton in 1923.
Compton derived the mathematical relationship between the shift in wavelength and the scattering angle of the X-rays by assuming that each scattered X-ray photon interacted with only one electron. He did reporting on experiments which verified his derived relation:
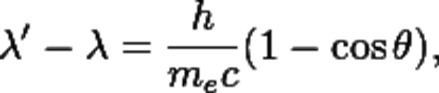 The quantity h⁄mec is known as the Compton wavelength of the electron; it is equal to 2.43×10−12 m. The wavelength shift λ′ − λ is at least zero (for θ = 0°) and at most twice the Compton wavelength of the electron (for θ = 180°).
The quantity h⁄mec is known as the Compton wavelength of the electron; it is equal to 2.43×10−12 m. The wavelength shift λ′ − λ is at least zero (for θ = 0°) and at most twice the Compton wavelength of the electron (for θ = 180°).
The effect is important because it demonstrates that light cannot be explained purely as a wave phenomenon. Thomson scattering, the classical theory of an electromagnetic wave scattered by charged particles, cannot explain low intensity shifts in wavelength. Light must behave as if it consists of particles to explain the low-intensity Compton scattering. Compton's experiment convinced physicists that light can behave as a stream of particle-like objects (quanta) whose energy is proportional to the frequency.
43. Annihilation.In physics, the word is used to denote the process that occurs when a subatomic particle collides with its respective antiparticle. Antiparticles have exactly opposite additive quantum numbers from particles, so the sums of all quantum numbers of the original pair are zero. Hence, any set of particles may be produced whose total quantum numbers are also zero as long as conservation of energy andconservation of momentum are obeyed. When a particle and its antiparticle collide, their energy is converted into a force carrier particle, such as a gluon, W/Z force carrier particle, or a photon. These particles are afterwards transformed into other particles.
Electron–positron annihilation.e− + e+ → γ + γ
When a low-energy electron annihilates a low-energy positron (antielectron), they can only produce two or more gamma ray photons, since the electron and positron do not carry enough mass-energy to produce heavier particles, and conservation of energy and linear momentum forbid the creation of only one photon. When an electron and a positron collide to annihilate and create gamma rays, energy is given off.
Proton-antiproton annihilation.When a proton encounters its antiparticle (and more generally, if any species of baryon encounters any species of antibaryon), the reaction is not as simple as electron-positron annihilation. Unlike an electron, a proton is a composite particle consisting of three "valence quarks" and an indeterminate number of "sea quarks" bound by gluons.
52. Calculation of neutron dose. Neutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce radiation.
In health physics neutron radiation is considered a fourth radiation hazard alongside the other types of radiation. Another, sometimes more severe hazard of neutron radiation, is neutron activation, the ability of neutron radiation to induce radioactivity in most substances it encounters, including the body tissues of the workers themselves. This occurs through the capture of neutrons by atomic nuclei, which are transformed to another nuclide, frequently a radionuclide.
We can calculate this with formula below:
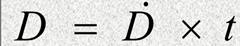
D-is the radiation dose;D.-radiation rate;t-is the exposure time;Absorbed dose from neutrons;• Elastic scatter (higher energies);• Capture (thermal neutrons);Thermal neutrons

Ф= thermal neutron fluence (n/cm2); N = atom density (cm-3); σ = capture cross section (for each element); E = energy from capture reaction; ρ = tissue density.
Absorbed dose from fast neutronsScattering: assume average energy lost is ½ Emax
| <== попередня сторінка | | | наступна сторінка ==> |
| Boron lined proportional detectors | | |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |