
РЕЗОЛЮЦІЯ: Громадського обговорення навчальної програми статевого виховання
ЧОМУ ФОНД ОЛЕНИ ПІНЧУК І МОЗ УКРАЇНИ ПРОПАГУЮТЬ "СЕКСУАЛЬНІ УРОКИ"
ЕКЗИСТЕНЦІЙНО-ПСИХОЛОГІЧНІ ОСНОВИ ПОРУШЕННЯ СТАТЕВОЇ ІДЕНТИЧНОСТІ ПІДЛІТКІВ
Батьківський, громадянський рух в Україні закликає МОН зупинити тотальну сексуалізацію дітей і підлітків
Відкрите звернення Міністру освіти й науки України - Гриневич Лілії Михайлівні
Представництво українського жіноцтва в ООН: низький рівень культури спілкування в соціальних мережах
Гендерна антидискримінаційна експертиза може зробити нас моральними рабами
ЛІВИЙ МАРКСИЗМ У НОВИХ ПІДРУЧНИКАХ ДЛЯ ШКОЛЯРІВ
ВІДКРИТА ЗАЯВА на підтримку позиції Ганни Турчинової та права кожної людини на свободу думки, світогляду та вираження поглядів
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Lection 11. Role of Melatonin in the Regulation of Human Circadian Rhythms and Sleep
The circadian rhythm of pineal melatonin is the best marker of internal time under low ambient light levels. The endogenous melatonin rhythm exhibits a close association with the endogenous circadian component of the sleep propensity rhythm. This has led to the idea that melatonin is an internal sleep 'facilitator' in humans, and therefore useful in the treatment of insomnia and the readjustment of circadian rhythms. There is evidence that administration of melatonin is able: (i) to induce sleep when the homeostatic drive to sleep is insufficient; (ii) to inhibit the drive for wakefulness emanating from the circadian pacemaker; and (iii) induce phase shifts in the circadian clock such that the circadian phase of increased sleep propensity occurs at a new, desired time. Therefore, exogenous melatonin can act as soporific agent, a chronohypnotic, and/or a chronobiotic. We describe the role of melatonin in the regulation of sleep, and the use of exogenous melatonin to treat sleep or circadian rhythm disorders.
Endogenous melatonin and the circadian sleep-wake cycle and thermoregulation
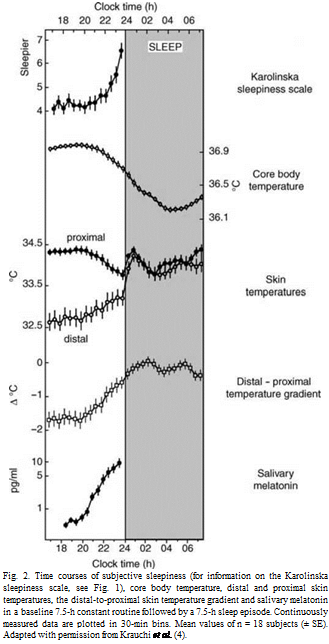
Under entrained conditions, the phase relationship between the endogenous circadian rhythm of melatonin and the sleep-wake cycle is such that during the usual 16-h waking day, stable levels of neurobehavioural function can be maintained. This occurs because the circadian pacemaker opposes the decrements in neurobehavioural function associated with increased homeostatic drive for sleep accumulating with sustained wakefulness. Extension of the wake episode into the biological night (i.e. past the evening rise of melatonin) is associated with marked decrements in neurobehavioural function, because the circadian pacemaker no longer opposes the wake-dependent deterioration but, instead, promotes sleep at this circadian phase. Thus, shortly after habitual bedtime, a sharp increase in subjective sleepiness and its electrophysiological correlates occurs (Fig. 1). This latter phenomenon has been referred to as ‘the opening of the sleep gate’. In parallel, the entire thermoregulatory cascade (i.e. decrease in heat production and increase in heat loss leading to decrease in core body temperature) starts with the rise in endogenous melatonin levels in the evening (Fig. 2). Melatonin onset seems to be the hormonal signal timing the rise in blood flow in distal skin regions and hence heat loss, the degree of which (measured by the distal-proximal skin temperature gradient) is the best physiological predictor for the rapid onset of sleep.
The association of sleep with the melatonin rhythm has been confirmed in blind people in whom the circadian pacemaker is not entrained and in sighted subjects with non 24-sleep-wake cycle syndrome. Even more impressive are the results obtained from studies using the forced desynchrony protocol to separate out circadian- and wake-dependent components of behaviour. The daily circadian increase in melatonin secretion coincides with a decrease in wake episodes during scheduled sleep episodes (Fig. 3). Sleep consolidation gradually deteriorates during that phase of the circadian cycle with low melatonin production. Electroencephalogram (EEG) activation during wakefulness is also timed at a specific phase relative to the circadian melatonin rhythm.
These carefully controlled experiments clearly show that the circadian pacemaker drives the rhythms of melatonin synthesis, thermoregulation, sleep consolidation and EEG activation during wakefulness. There may also be feedback from the pineal gland to both the circadian pacemaker and thermoregulatory centers in the hypothalamus. The interpretation is that melatonin weakens the circadian signal from the suprachiasmatic nuclei (SCN), promoting heat loss which induces sleepiness via the preoptic area of the anterior hypothalamus. Any effect of melatonin on sleepiness and sleep must be relative rather than absolute, however, because individuals who secrete no melatonin at all seem to sleep normally.
Effects of exogenous melatonin on sleep and thermoregulation

 |
There is ample evidence for a close temporal relationship between the melatonin secretory phase and thermoregulation and circadian sleep propensity. However, is melatonin causally involved in sleep and thermoregulatory mechanisms? Is it necessary, or sufficient? Initially, the answer is no. The ability to sleep is still possible in the absence of detectable endogenous melatonin during the day, or in tetraplegic patients, and only a moderate incidence of sleep disturbance has been reported in pinealectomized patients. Absolute melatonin production (which varies enormously between individuals) does not correlate with sleep quality in the elderly or elderly sleep-maintenance insomniacs. To our knowledge, whether thermoregulatory mechanisms are changed in low melatonin secretors, pinealectomized patients and in traumatic spinal cord injury subjects whose melatonin production is absent, has not been investigated. At least in patients with spinal injuries above T6, thermoregulation is impaired because of the interruption of neuronal pathways to and from the hypothalamus.
By contrast, numerous laboratory studies under stringent conditions clearly demonstrate that administration of melatonin acutely affects sleep and thermoregulation in humans. Therefore, on second glance, the answer to the question of the causal role of melatonin would be yes. Exogenous melatonin elicits all the physiological effects which occur in the evening during endogenous melatonin secretion. Indeed, exogenous melatonin is most effective when endogenous levels are low during the biological day. It elicits time-dependent soporific effects, which have been corroborated with electrophysiological measures of sleepiness such as electroencephalographic theta activity during wakefulness 
 |
.
The soporific effect is paralleled by a time- and dose- dependent hypothermic action, mediated by an increase in heat loss. In an experiment where we blocked this natural evening increase in heat loss, subjective sleepiness, and melatonin secretion by light exposure, we could show that melatonin replacement (5 mg) acutely recovered the evening increase in heat loss, subjective sleepiness and also theta activity in the waking EEG. Together, these data suggest a causal relationship between melatonin and sleepiness, probably mediated by thermoregulatory mechanisms. This supports the hypothesis that the onset of melatonin secretion might contribute to the rise in sleepiness and sleep propensity that occurs in the evening. It remains to be established whether exogenous melatonin acts via the SCN and the thermoregulatory centres in the preoptic area of the anterior hypothalamus, or whether it is a peripheral effect on receptors in the arterio-venous anastomoses, or both.
Quantitative analysis of the sleep EEG based on the fast Fourier transform (FFT) has revealed that 5 mg melatonin, given shortly before a daytime sleep episode, suppresses low EEG components and increases EEG activity in the sleep-spindle frequency range. Interestingly, similar changes in the sleep EEG spectra occur in sleep during the melatonin secretory phase (biological night) when compared with sleep occurring outside the melatonin secretory phase (biological day). Two different experimental paradigms have revealed this: a forced desynchrony protocol and a nap protocol. The two top panels in Fig. 4 illustrate the similarity in relative EEG power spectra during night-time sleep (high endogenous melatonin levels) and during daytime sleep after melatonin administration (5 mg). The bottom panel shows EEG power density during night-time sleep after melatonin ingestion (5 mg), which did not differ significantly from a baseline placebo night-time sleep recording. These studies suggest that as soon melatonin is secreted (biological night), an extra dose of melatonin (5 mg) has no further effect on the spectral composition of the sleep EEG. Sleep spindles are presumably generated in the nucleus reticularis of the thalamus and are enhanced by GABAA-receptor agonists. The effects of melatonin are, to some extent, and to a much lower degree, similar to the changes induced by GABAA agonists such as benzodiazepine hypnotics. Both agents increase EEG activity in the frequency range of sleep spindles. However, given that melatonin’s action (3mg) on sleep EEG spectra was not blocked by flumanzenil (10 mg), a GABAA antagonist which blocks benzodiazepine effects, the mechanisms may be dissimilar.
Most studies on the role of melatonin in sleep have been confined to classical sleep scoring analyses. Therefore, replication of the above mentioned FFT findings are required. The most consistent effect found in those studies was that sleep latency was shorter after melatonin, even at rather low doses. On the other hand, sleep consolidation or sleep efficiency was not affected by night-time melatonin administration whereas, during daytime, an improvement in sleep efficiency could be found. Recent data from a forced desynchrony protocol, where melatonin was given to healthy young adults across a full range of circadian phases, confirm that exogenous melatonin can only increase sleep efficiency outside the time window of its normal production.
Similar findings come from an extended sleep protocol. Chronic administration of melatonin in a slow-release formulation during a 16-h sleep opportunity beginning at 16.00h resulted in a redistribution of sleep so that sleep efficiency during the first half of the sleep opportunity was substantially higher during melatonin treatment compared to placebo. These two recent studies provide strong support for the hypothesis that exogenous melatonin attenuates the wake-promoting signal of the endogenous circadian pacemaker, allowing for increased sleep efficiency at circadian phases corresponding to the habitual wake episode. In summary, endogenous melatonin has an important role in the circadian regulation of sleep (sleep timing), and exogenous melatonin exerts effects on the main characteristics of human sleep (i.e. slow waves, sleep spindles, sleep latency and sleep consolidation).
Effects of exogenous melatonin on circadian rhythms
In a variety of animal species melatonin is a ‘zeitgeber’, which induces phase shifts and entrainment of the circadian clock underlying the expression of many 24-h rhythms. Melatonin is also a major entraining signal for the circadian systems of fetal and neonatal mammals. Daily exposure to circulating melatonin allows fetuses to be synchronized with each other and with their mother long before they can directly perceive the environmental light/dark cycle on their own. Overall, the animal literature clearly supports the role of melatonin as a chronobiotic.
In sighted humans, under real life conditions, exogenous melatonin may not be able to sufficiently override the most important ‘zeitgeber’ light to produce a robust consistent, statistically significant phase-shifting effect on the day after administration. An elegant approach to elaborate phase-shifting capacities of melatonin has been in totally blind people. In such individuals, light/dark information fails to reach the endogenous circadian pacemaker. In a proportion of these individuals, circadian rhythms (e.g. melatonin and core body temperature) do not synchronize with the environment and free run usually with a period length >24h. Recently, it was demonstrated that melatonin treatment (0.510 mg) can entrain the circadian system (melatonin or cortisol rhythms) of some free-running blind people if initiated at an appropriate time relative to internal time. In addition, melatonin can stabilize sleep/wake timing even without entraining the circadian system. As for the ‘zeitgeber’ light, a number of studies have begun to delineate a phase-response curve (PRC) for melatonin. A ‘classic’ PRC measures phase shifts following a single exposure to a ‘zeitgeber’ under free-running conditions. Repeated doses of melatonin do yield a PRC, with the direction of the phase shift dependent on the time of administration. Unfortunately, in these studies, light, a much more powerful ‘zeitgeber’ on the human circadian pacemaker than melatonin, was not controlled, or was of too high an intensity to provide unmasked melatonin onset times. However, in a double-blind, placebo-controlled, crossover study in which subjects were studied under dim light and constant posture conditions, we and others showed that a single melatonin dose at 18.00h induced an advance of the circadian nocturnal decline in core body temperature, heart rate and the dim-light melatonin onset as assessed on the second day (more than 24 h) after melatonin administration. In the same study, an earlier offset of sleep was observed in the second night after melatonin administration. Because this sleep episode was initiated 29 h after melatonin administration, these effects have been interpreted as reflecting a phase advance of the circadian timing system similar to the effects of bright light exposure in the morning hours. Interestingly, in our similarly designed constant routine study of 5 mg melatonin given in the morning (07.00 h), no evidence for a phase delay in the above circadian rhythms was found. This means that either we have missed the appropriate timing for a phase delay [selected according to Zaidan et al. which might be later in the morning (i.e. after endogenous melatonin levels had declined) or that the dose was too high and overlapped into the phase advance portion of the hypothetical PRC. To our knowledge, there is only one other randomized, double-blind, placebo-controlled trial under controlled light conditions that investigated the capacity of melatonin to induce phase delays. The authors reported delays in the onset of the melatonin secretory phase after administration of melatonin at 07.00h; however, no significant phase shift in the offset of secretion could be determined. These data suggest that it is difficult to phase delay human circadian rhythms by exogenous melatonin. In fact, the evidence that melatonin has phase-delaying effects (> 30 min) in humans is based on very limited data. It appears that the precise timing of melatonin administration is crucial in order to exploit its chronobiotic properties. Therefore, there is an urgent need for a complete PRC for a single dose of melatonin carried out under stringently controlled laboratory conditions.
Implications for the treatment of insomnia and circadian rhythms disorders
The soporific and chronobiotic properties of melatonin make it an optimal candidate for treating sleep, in addition to circadian rhythm disorders. In our view, the most successful attempt to treat insomnia and changes in circadian phase position by melatonin has been carried out in free-running blind people. Optimal melatonin treatment in those people should utilize not only its soporific effects by administration close to the desired bedtime, but also its chronobiotic properties, in order to entrain sleep/wake behaviour. Another promising patient group are elderly patients with insomnia. However, compared to the excellent studies in the blind, randomized, double-blind, placebo-controlled trials are rare (n = 6). In those six studies, the results of melatonin treatment (0.5-6 mg) administered before bedtime were not consistent: sleep latency decreased in four studies and sleep efficiency improved in three studies, whereas subjective sleep quality did not improve in any of the studies. Reviewing 78 articles on melatonin treatment in elderly insomniacs, Olde Rikkert and Rigaud concluded that melatonin is most effective in elderly insomniacs who chronically use benzodiazepines and/or with documented low melatonin secretion during sleep.
Abnormal timing of sleep with respect to circadian phase occurs in the delayed sleep phase syndrome (DSPS), in which sleep occurs at a delayed clock time relative to the light/dark cycle, social, work, and family demands. In the first use of melatonin in patients with DSPS, it was found that, when administered 5 h before sleep onset for a period of 4 weeks, melatonin (5 mg) advanced sleep onset and wake times compared to placebo. A more recent study confirmed this, showing that melatonin induces an advance in sleep onset in patients with DSPS, and is most effective in DSPS patients with shorter habitual sleep time and later clinical onset.
The first application of melatonin using chronobiological principles was to alleviate the perceived effects of jet lag. There have been many placebo-controlled and uncontrolled studies that have recently been summarized by Cochrane. This stringent analysis concludes that nine of 10 trials of melatonin, taken close to the target bedtime at destination, decreased jet lag symptoms arising after flights crossing five or more time zones. One difficulty in using melatonin for jet lag is that its use may require administration at times when it will have undesired soporific properties.
There is also a great interest in whether melatonin can facilitate phase-shifting in night-shift workers; however, few studies have measured such phase shifts. In two laboratory studies, circadian rhythms were measured before and after a large shift in the sleep/ wake schedule. Melatonin (5 mg) was administered during the phase-advance portion of the PRC and produced larger circadian phase shifts than placebo. In the other study, subjects took 4 mg melatonin (or placebo) before and during their daytime sleep and melatonin did not produce a larger phase delay than placebo. In a night-shift field study, melatonin produced larger circadian phase shifts than placebo in only seven of the first 24 subjects studied. Overall, these studies do not provide strong evidence that melatonin can help phase shift the circadian rhythms of night-shift workers, in particular, when comparing its action as being less strong than exposure to light. One problem has been the lack of control over time of melatonin administration, and of the subjects’ sleep schedules. In a recent study where the timing of melatonin administration, the sleep/ wake schedule and, to some extent, the light/dark cycle could be controlled in a field setting, melatonin produced larger phase advances than placebo in the circadian rhythms of melatonin and core body temperature. Additional caution is required in this setting to avoid the soporific effects of melatonin during work requiring vigilance, or driving home after the shift.
Conclusions
A remarkably tight association between the circadian rhythms of melatonin and sleep propensity and thermoregulation has been described in humans. Together with the observation that daytime administration of melatonin increases sleep propensity and decreases core body temperature via heat loss induction, this suggests that melatonin has direct effects on sleep-inducing thermoregulatory mechanisms. The circadian rhythm of melatonin secretion may be part of the pathway by which the circadian pacemaker drives the circadian rhythm of sleep propensity, sleep structure and core body temperature.
There is clear evidence that melatonin induces phase shifts, particularly phase advances, in human circadian rhythms, and precisely-timed melatonin can be useful for the treatment of insomnia related to jet lag or shift work. However, there is the need for a detailed PRC to single doses of melatonin in humans under stringently-controlled laboratory conditions. Furthermore, further studies are required using physiological doses and/or delivery systems that generate ‘natural’ melatonin profiles, while at the same time scheduling sleep at a variety of circadian phases to establish the role of melatonin in the circadian regulation of sleep. In summary, the combined circadian and soporific properties of melatonin make it an attractive tool not only for basic circadian/ sleep research, but also as an attractive candidate for the treatment of sleep disorders related to inappropriate circadian timing.
Читайте також:
- A Short Course in Human Relations
- Art-collections.
- B. Express your personal point of view regarding the challenges that are in store for us in terms of preserving the environment and saving the humankind in the near future.
- Civil Aviation Security Regulations
- Creating Selection Lists
- Data Collection and Analysis
- Data Collection Methods for Market Research
- Human Factor and Aviation Safety Problems
- Human gene therapy
- Humanities
- Humans vs. the environment - A thought experiment
- Inflection
| <== попередня сторінка | | | наступна сторінка ==> |
| | |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |