
РЕЗОЛЮЦІЯ: Громадського обговорення навчальної програми статевого виховання
ЧОМУ ФОНД ОЛЕНИ ПІНЧУК І МОЗ УКРАЇНИ ПРОПАГУЮТЬ "СЕКСУАЛЬНІ УРОКИ"
ЕКЗИСТЕНЦІЙНО-ПСИХОЛОГІЧНІ ОСНОВИ ПОРУШЕННЯ СТАТЕВОЇ ІДЕНТИЧНОСТІ ПІДЛІТКІВ
Батьківський, громадянський рух в Україні закликає МОН зупинити тотальну сексуалізацію дітей і підлітків
Відкрите звернення Міністру освіти й науки України - Гриневич Лілії Михайлівні
Представництво українського жіноцтва в ООН: низький рівень культури спілкування в соціальних мережах
Гендерна антидискримінаційна експертиза може зробити нас моральними рабами
ЛІВИЙ МАРКСИЗМ У НОВИХ ПІДРУЧНИКАХ ДЛЯ ШКОЛЯРІВ
ВІДКРИТА ЗАЯВА на підтримку позиції Ганни Турчинової та права кожної людини на свободу думки, світогляду та вираження поглядів
- Гідрологія і Гідрометрія
- Господарське право
- Економіка будівництва
- Економіка природокористування
- Економічна теорія
- Земельне право
- Історія України
- Кримінально виконавче право
- Медична радіологія
- Методи аналізу
- Міжнародне приватне право
- Міжнародний маркетинг
- Основи екології
- Предмет Політологія
- Соціальне страхування
- Технічні засоби організації дорожнього руху
- Товарознавство продовольчих товарів
Тлумачний словник
Авто
Автоматизація
Архітектура
Астрономія
Аудит
Біологія
Будівництво
Бухгалтерія
Винахідництво
Виробництво
Військова справа
Генетика
Географія
Геологія
Господарство
Держава
Дім
Екологія
Економетрика
Економіка
Електроніка
Журналістика та ЗМІ
Зв'язок
Іноземні мови
Інформатика
Історія
Комп'ютери
Креслення
Кулінарія
Культура
Лексикологія
Література
Логіка
Маркетинг
Математика
Машинобудування
Медицина
Менеджмент
Метали і Зварювання
Механіка
Мистецтво
Музика
Населення
Освіта
Охорона безпеки життя
Охорона Праці
Педагогіка
Політика
Право
Програмування
Промисловість
Психологія
Радіо
Регилия
Соціологія
Спорт
Стандартизація
Технології
Торгівля
Туризм
Фізика
Фізіологія
Філософія
Фінанси
Хімія
Юриспунденкция
Добування ацетилену
C
Алкени
С
Добування циклоалканів
·  з дигалогенопохідних:
з дигалогенопохідних:
СН2 – CH2 – CH2 – СН2 + Zn → + ZnCl2
׀ ׀
Cl Cl
 CH3 CH3
CH3 CH3
 ׀
׀
СН2 – CH2 – CH – СН2 + Zn → + ZnCl2
׀ ׀
Cl Cl
· гідрогенізація аренів:


 Pt
Pt
+ 3Н2 →
Використання циклоалканів у медицині
 |
Циклопропан –інгаляційний наркоз.
· це ненасичені вуглеводні з відкритим ланцюгом, в якому між атомами С є один подвійний зв’язок, інші – одинарні ( олефіни, етиленові вуглеводні).
Загальна формула СnH2n.
Гомологічний ряд алкенів:
С2Н4 етен (етилен) СН2 = СН2
С3Н6 пропен (пропілен) СН2 = СН – СН2
С4Н8 бутен СН2 = СН – СН2 – СН3
CH3 – CH = CH – СН3
С5Н10 пентен
С6Н12 гексен
С7Н14 гептен
С8Н16 октен
С9Н18 нонен
С10Н20 децен
Ізомерія:
–за вуглецевим скелетом СН2 = СН – СН2 – СН3 СН2 = С – СН3
׀
1 – бутенСН3
2 – метилпропен
– за положенням подвійного зв’язку СН2 = СН – СН2 – СН3 1 – бутен
СН3 – СН = СН – СН3 2 – бутен
– міжкласова ізомерія (з циклоалканами)
СН2 = СН – СН2 – СН3 1 – бутен

циклобутан
– геометрична ізомерія
CH3 – CH = CH – СН3 бутен – 2
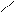
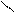
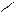
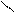 Н Н Н СН3
Н Н Н СН3

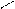

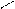 С = С С = С
С = С С = С
Н3С СН3 Н3С Н
цис – бутен – 2
транс – бутен – 2
Електронна будова:
· тип гібридизації sp2
· валентний кут 120°
· форма молекули площинна
· хімічні зв’язки – подвійний, 1 σ та 1 π
· довжина зв’язку С = С0,134 нм
Фізичні властивості: С2 – С4гази
С5 – С15рідини
С16 і більше –тверді
Без кольору та запаху, нерозчинні у воді.
Хімічні властивості етилену
1 – горіння:C2H4 + 3O2 → 2CO2 + 2H2O
1000°С
 2 – розклад:C2H4 → 2C + 2H2
2 – розклад:C2H4 → 2C + 2H2
– приєднання: а) гідрогенізація СН2 = СН2 + Н2 → СН3 – СН3
б) галогенування СН2 = СН2 + Cl2 → СН2 – СН2
׀ ׀
Cl Cl
в) гідрогалогенування СН2 = СН2 + НCl → СН3 – СН2
׀
Cl
г) гідратація СН2 = СН2 + НОН → СН3 – СН2
׀
 ОН
ОН
– окислення (реакція Вагнера): СН2 = СН2 + [O] + НОН → СН2 – СН2
׀ ׀
 kat ОН OH
kat ОН OH
– полімеризація: nСН2 = СН2 → (– СН2 – СН2 –)n
Хімічні властивості пропілену
1 – горіння: 2C3H6 + 9O2 → 26CO2 + 6H2O
1000°С
 2 – розклад:C3H6 → 3C + 3H2
2 – розклад:C3H6 → 3C + 3H2
– приєднання:
а) гідрогенізація СН3 – СН = СН2 + Н2 → СН3 – СН2 – СН3
б) галогенування СН3 – СН = СН2 + Cl2 → СН3 – СН – СН2
׀ ׀
Cl Cl
в) гідрогалогенування СН3 – СН = СН2 + НCl → СН3 – СН – СН3
׀
Cl
г) гідратація СН3 – СН = СН2 + НОН → СН3 – СН – СН3
׀
ОН
| |||
 |
–окислення (реакція Вагнера):
СН3 – СН2 = СН2 + [O] + НОН → СН3 – СН – СН2
׀ ׀
 kat ОН OН
kat ОН OН
– полімеризація: nСН = СН2 → (– СН – СН2 –)n
׀׀
СН3 СН3
|
яяя
|
Алкадієни
· це ненасичені вуглеводні з відкритим ланцюгом, в якому між атомами С є два подвійних зв’язка, інші – одинарні (дієни).
Загальна формула СnH2n–2.
Гомологічний ряд алкадієнів:
С3Н4 пропадієн СН2 = С = СН2
С4Н6 бутадієн СН2 = СН – СН = СН2
CH2 = C = CН – СН3
С5Н8 пентадієн
С6Н10 гексадієн
С7Н12 гептадієн
С8Н14 октадієн
С9Н16 нонадієн
С10Н18 декадієн
Ізомерія:
– за вуглецевим скелетом
СН2 = СН – СН = СН – СН3 1,3 – пентадієн
СН2 = С – СН = СН2 2 – метил – 1,3 – бутадієн
׀
СН3
– міжкласова ізомерія (з алкінами)
СН2 = СН – СН = СН2 1,3 – бутадієн
СН ≡ С – СН2 – СН3 1 – бутин
Електронна будова:
·  тип гібридизації sp2
тип гібридизації sp2
· валентний кут 120°
·  форма молекули площинна
форма молекули площинна
· хімічні зв’язки – 2 подвійних (1 σ та 1 π).
Види алкадієнів
· кумуленові –подвійні зв’язки розташовані поруч:
СН2 = С = СН2
CH2 = C = CН – СН3
· ізольовані– подвійні зв’язки розділені більш, ніж одним σ – зв’язком:
СН2 = СН – СН2 – СН = СН2
СН2 = СН – СН2 – СН = СН – СН3
Хімічні властивості не відрізняються від властивостей алкенів, з урахуванням того, що в реакцію можуть вступати два незалежних подвійних зв’язка.
· спряжені– подвійні зв’язки розділені одним σ – зв’язком:
СН2 = СН – СН = СН2
СН2 = СН – СН = СН – СН3
В молекулах таких дієнів утворюється єдина π – хмара:







 ≡
≡
≡
Відстань між атомами частково усереднюється: довжина подвійних зв’язків більша, а одинарного – менша, ніж звичайно:
0,135нм 0,135нм




 СН2 СН СН СН2
СН2 СН СН СН2
0,148нм
Спряжена система реагує як єдине ціле.
Хімічні властивості 1,3 – бутадієну

– приєднання:
а) гідрогенізація СН2 = СН – СН = СН2 + Н2 → СН3 – СН = СН – СН3
б) галогенування СН2 = СН – СН = СН2 + Сl2 → СН2 – СН = СН – СН2
׀ ׀
Cl Cl
в) гідрогалогенування СН2 = СН – СН = СН2 + НCl → СН3 – СН = СН – СН2
׀
 kat Cl
kat Cl
– полімеризація: nСН2 = СН – СН = СН2 → (– СН2 – СН = СН – СН2 –)n
синтетичний каучук
Хімічні властивості ізопрену
(2 – метил – 1,3 – пентадієну)

– приєднання:
а) гідрогенізація СН2 = С – СН = СН2 + Н2 → СН3 – С = СН – СН3
׀ ׀
СН3 СН3
б) галогенування СН2 = С – СН = СН2 + Сl2 → СН2 – С = СН – СН2
׀ ׀ ׀ ׀
СН3 Cl СН3 Cl
в) гідрогалогенування СН2 = С – СН = СН2 + НCl → СН3 – С = СН – СН2
׀ ׀ ׀
 СН3 kat СН3 Cl
СН3 kat СН3 Cl
– полімеризація: nСН2 = С – СН = СН2 → (– СН2 – С = СН – СН2 –)n
׀ ׀
СН3 СН3
природний каучук
Добування алкадієнів
1 – каталітична дегідрогенізація:
Cr2O3, Al2O3
 СН3 – CH2 – CH2 – СН3 СН2 = СН – СН = СН2 + 2Н2
СН3 – CH2 – CH2 – СН3 СН2 = СН – СН = СН2 + 2Н2
продукт переробки нафти 600°C
Cr2O3, Al2O3
 СН3 – CH – CH2 – СН3 СН2 = С – СН = СН2 + 2Н2
СН3 – CH – CH2 – СН3 СН2 = С – СН = СН2 + 2Н2
׀ 600°C׀
СН3 ZnO MgOСН3
 2 – синтез Лебедєва:2СН3 – СН2ОН СН2 = СН – СН = СН2 + Н2 + 2Н2О
2 – синтез Лебедєва:2СН3 – СН2ОН СН2 = СН – СН = СН2 + Н2 + 2Н2О
Алкіни
· це ненасичені вуглеводні з відкритим ланцюгом, в якому між атомами С є один потрійний зв’язок, інші – одинарні ( ацетиленові вуглеводні).
Загальна формула СnH2n–2.
Гомологічний ряд алкінів:
С2Н2 етин (ацетилен) СН ≡ СН
С3Н4 пропін СН ≡ С – СН2
С4Н6 бутин СН ≡ С – СН2 – СН3
CH3 – C ≡ C – СН3
С5Н8 пентин
С6Н10 гексин
С7Н12 гептин
С8Н14 октин
С9Н16 нонін
С10Н18 децин
Ізомерія:
–за вуглецевим скелетом СН ≡ С – СН2 – СН2 – СН3 1 – пентин
СН ≡ С – СН – СН3 3 – метилбутин
׀
СН3
– міжкласова ізомерія (з алкадієнами) СН ≡ С – СН2 – СН3 1 – бутин
СН2 = СН – СН = СН2 1,3 – бутадієн
Електронна будова:
· тип гібридизації sp
· валентний кут 180°
· форма молекули лінійна
· хімічні зв’язки – потрійний, 1 σ та 2 π
· довжина зв’язку С ≡ С0,120 нм
Фізичні властивості: С2 – С4гази
С5 – С15рідини
С16 і більше –тверді
Без кольору та запаху, нерозчинні у воді.

 |  | |||||||||||
 | ||||||||||||
 | ||||||||||||
| ||||||||||||
 | ||||||||||||
 | ||||||||||||
 | ||||||||||||
 |
СН ≡ СН + Н2 → СН2 = СН2
СН ≡ СН + 2Н2 → СН3 – СН3 ׀
б) галогенування
1 – карбідний спосіб:СaC2 + 2H2O → Ca(OH)2 + C2H2
Читайте також:
- Добування карбонільних сполук
- ДОБУВАННЯ КАРБОНОВИХ КИСЛОТ
- ДОБУВАННЯ КОРИСНИХ КОПАЛИН ВІДКРИТИМ СПОСОБОМ
- ДОБУВАННЯ КОРИСНИХ КОПАЛИН ВІДКРИТИМ СПОСОБОМ
- Добування толуолу
- Добування фенолу
- Добування чистих металів.
- Добування.
- Загальні способи добування металів.
- Класифікація методів практичного видобування знань
- Піднесення до степеня і добування кореня
| <== попередня сторінка | | | наступна сторінка ==> |
| Циклоалкани | | | Гомологи бензолу |
|
Не знайшли потрібну інформацію? Скористайтесь пошуком google: |
© studopedia.com.ua При використанні або копіюванні матеріалів пряме посилання на сайт обов'язкове. |